How to Get
Brekiya autoinjector
Brekiya autoinjector is exclusively available through two specialty pharmacies
You may be eligible to pay as little as $40 per carton*,†
*Restrictions apply.

†Each carton contains 4 single-dose autoinjectors.1
HOW DO I GET MY PRESCRIPTION?
1
Your healthcare provider will submit your prescription to one of two specialty pharmacies, and that pharmacy will call or text you within 2 business days.
2
The specialty pharmacy will confirm your insurance coverage. If there are any questions, they will call or text‡ you or your healthcare provider, as needed.
Some of the things they may need to confirm with you are:
- Date of birth
- Cell phone number
- E-mail address
- Insurance information
- Shipping address
NEED SUPPORT?
If you have not received a phone call from the pharmacy within 48 hours after visiting your healthcare provider or have any questions about your medication, please contact the specialty pharmacy directly by phone.
Be sure to answer any text message or calls from your specialty pharmacy to get your prescription filled.

Phone number: 888-618-4126

Phone number: 888-282-5166
‡Standard text messaging rates apply.

Eligible patients may be eligible for financial assistance for your Brekiya autoinjector prescription
Copay Program
Explore helpful resources
Review the access and educational materials below to better support you.
Brekiya autoinjector resources
Support you can count on
Explore resources and community connections that can help empower and assist you every step of the way along your journey.
< PREVIOUS PAGE
NEXT PAGE >
Expand
Collapse
Serious or potentially life-threatening reductions in blood flow to the brain or extremities due to interactions between dihydroergotamine (the active ingredient in Brekiya autoinjector) with strong CYP3A4 inhibitors (such as protease inhibitors and macrolide antibiotics) have been reported rarely. As a result, these medications should not be taken together.
Do not use Brekiya autoinjector if you:
- are taking medicines known as strong CYP3A4 inhibitors, such as: ritonavir, nelfinavir, indinavir, erythromycin, clarithromycin, ketoconazole, or itraconazole.
- have heart problems or a history of heart problems, or uncontrolled high blood pressure.
- have narrowing of blood vessels in your legs, arms, stomach, or kidneys (peripheral vascular disease).
- have sepsis, had vascular surgery, severe liver or kidney problems.
- are allergic to dihydroergotamine, ergot alkaloids, latex, or any of the ingredients in Brekiya autoinjector.
- have taken any of the following medicines in the last 24 hours: sumatriptan, frovatriptan, ergotamine or ergotamine-type medicines, almotriptan, naratriptan, zolmitriptan, eletriptan, or rizatriptan
- have taken any medicines that constrict your blood vessels or raise your blood pressure.
- have high blood pressure, liver problems, or kidney problems.
- have risk factors for heart disease, such as: high blood pressure, high cholesterol levels, smoke, are overweight, diabetes, family history of heart disease.
- are pregnant or plan to become pregnant. Brekiya autoinjector may cause preterm labor. Brekiya autoinjector should be avoided during pregnancy. Talk to your healthcare provider right away if you are pregnant or want to become pregnant.
- are breastfeeding or plan to breastfeed. Brekiya autoinjector may reduce breast milk supply and pass into your breast milk. Brekiya autoinjector may be harmful to your baby. Do not breastfeed your baby while taking Brekiya autoinjector and for 3 days after you use Brekiya autoinjector. Talk with your healthcare provider about the best way to feed your baby if you take Brekiya autoinjector.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Especially tell your healthcare provider if you take: sumatriptan, ergot-type medicine, saquinavir, nefazodone, fluconazole, grapefruit juice, zileuton, nicotine, propranolol or other medicines that can lower your heart rate, any medicines that can increase your blood pressure, selective serotonin reuptake inhibitors. These are not all of the medicines that could affect how Brekiya autoinjector works. Your healthcare provider can tell you if it is safe to take Brekiya autoinjector with other medicines.
How should I use Brekiya autoinjector?
- Certain people should take their first dose of Brekiya autoinjector in their healthcare provider’s office or in another medical setting.
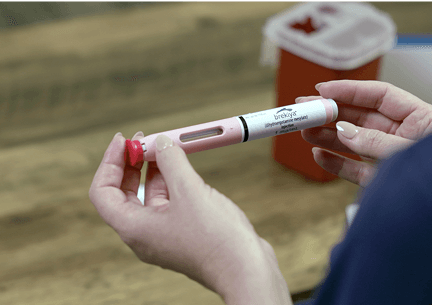
- Brekiya autoinjector is for injection under the skin (subcutaneous) only.
- Use Brekiya autoinjector exactly as your healthcare provider tells you to use it.
- Each autoinjector contains one dose (1 mg).
- If your headache comes back after the first complete dose, you may give yourself up to 2 more doses as needed. Wait at least one hour between doses.
- Do not inject more than 3 doses (3 mg) of Brekiya autoinjector in a 24‑hour period or 6 doses (6 mg) in a 1‑week (7 day) period.
What are the possible side effects of Brekiya autoinjector?
Brekiya autoinjector can cause serious side effects, including:
- Heart attack and other heart problems. Heart problems may lead to death. Stop taking Brekiya autoinjector and get emergency medical help right away if you have any symptoms of a heart attack, such as: numbness or tingling in your fingers and toes, muscle pain or cramps in your arms and legs, weakness in your legs, temporary speeding or slowing of your heart rate, or swelling or itching. Brekiya autoinjector is not for people with risk factors for heart disease unless a heart exam is done and shows no problem.
- Stroke. Stop using Brekiya autoinjector and get emergency medical help right away if you have any of the symptoms of a stroke.
- Changes in color or sensation in your fingers and toes (Raynaud’s syndrome).
- Stomach and intestinal problems.
- Increased blood pressure.
- Medicine overuse headache. Some people who use too much Brekiya autoinjector may make their headaches worse. If your headaches get worse, your healthcare provider may decide to stop your treatment with Brekiya autoinjector.
- Preterm labor.
- Tissue changes (fibrotic complications). Inflammation and fiber-like tissue that is not normal (fibrosis) can occur around the lungs and stomach.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all of the possible side effects of Brekiya autoinjector. Call your healthcare provider for medical advice about side effects.
- Store at room temperature between 68°F to 77°F (20°C to 25°C). Do not refrigerate or freeze.
- Protect Brekiya autoinjector from light.
- Keep Brekiya autoinjector in the original pack until ready to use.
Brekiya autoinjector is not used to prevent migraine or used to treat other types of headaches such as hemiplegic migraines (that make you unable to move on one side of your body) or basilar migraines (rare form of migraine with aura). It is not known if Brekiya autoinjector is safe and effective in children.
Please see full Prescribing Information, including Boxed Warning, Medication Guide, and Instructions for Use.
References: 1. Brekiya [package insert]. Bridgewater, NJ: Amneal Pharmaceuticals LLC; 2025.